In biomedicine, skim-reading is out of the question: every page requires poring over, which may take up to five minutes. Formulas, tables, charts, and the principles behind a trial warrant special attention, since patient safety is directly at stake. As a result, an expert may spend up to 25 hours or even more working on a single application. For example, the LEC at the National Medical Research Center of Oncology named after N.N. Petrov (NMRC) reviews 20 to 50 such applications every year, requiring vast amounts of effort, focus, knowledge, and time.
How Yandex’s neural networks helped doctors speed up new treatment method development
The NMRC of Oncology named after N.N. Petrov started using a YandexGPT-based solution to process applications on clinical trial ethical approvals. Now the initial document verification takes just a few minutes as opposed to a few weeks.
Local ethics committees: what they are and how they work
A local ethics committee (LEC) is an autonomous expert body at a healthcare organization deciding whether or not a human clinical trial is viable. The committee makes sure the participation is safe for patients, the ethical standards are met, and the subject selection is fair. A global healthcare practice, it became an official part of Russia’s legislation at the beginning of the 2000s.
An LEC is comprised of at least five experts: physicians and science center employees, at least one third-party representative, and at least one person with no medical background, ensuring the evaluation is independent and unbiased. Meetings take place on a regular basis, typically monthly. With more engagement of a medical organization into biotechnological innovation, the workload for the respective LEC is higher, which requires such an organization to work in a swifter and more accurate way.
Over 1,800 organizations conduct clinical trials for medical drugs in Russia, and almost each one has its own LEC. In case of multi-organization trials, multiple LECs at such organizations are simultaneously responsible for examining the same data without being able to properly synchronize their efforts or exchange critical information. This entails effort overlaps and increases the workload. In 2024 alone, Russia’s Ministry of Health issued 628 clinical trial permits, which generated a huge amount of documentation for LECs to examine.
The committees review applications from pharmaceutical companies and healthcare organizations regarding innovative solutions, clinical trials for medical drugs and items, and new treatments. Every application is a batch of documents spanning over hundreds of pages.
LECs liaise with clinical trial sponsors, research teams, healthcare organization managers, trial subjects, and regulatory bodies. LECs are publicly accessible bodies that must show irreproachable transparency.
Numerous document types undergo expert review: clinical trial sheets and their amendments, descriptions of drugs, medical items, or treatments under review, researchers’ summaries, informed consent forms for participants, and various supporting documents.
LECs receive documents in both paper and electronic form. However, most committees lack a full-fledged electronic document flow. They often have to deal with low-resolution PDF files, excessive copies, duplication, and no version management. This complicates the reviewing process and increases the risk of error. The complete cycle of reviewing and agreeing upon a documentation batch regarding a new clinical trial for a drug takes an average of over 50 days.
Project idea and members
To streamline its LEC’s operations using cutting-edge technology, staff at the National Medical Research Center of Oncology named after N.N. Petrov developed their own software prototype and contacted Yandex Cloud Social Tech. The idea of this project arose out of a practical demand research centers are faced with: too much time is spent on routine and repetitive actions, while delays in decision-making may have severe consequences for patients and drug inventors alike.
The biggest potential danger such delays pose when launching a clinical trial is the time patients lose waiting for access to a treatment that may prove critical. For patients with severe and rapidly progressing diseases, this waiting time may come at a price that is too high. Beyond that, every week of delay also means considerable financial loss; trial launch hold-ups are estimated to cost tens of millions of rubles in case the drug enters the market belatedly.
“When they say to a patient, ‘You have a chance but will have to wait a couple of months until the documents are ready,’ this brings very little hope. This person just wants to live on, after all. If, however, the treatment starts in a week, that is an opportunity taken. We are doing everything to increase the number of such taken opportunities and decrease that of delays.”
Yandex Cloud Social Tech suggested a solution architecture, helped find a sponsor for the project, and provided technologies adjusted for the tasks at hand. The sponsor was Raft, an integration company that develops products using LLMs and cloud services.
How the solution works
An LEC secretary uploads document scans to a web-based service, and YandexGPT 5 Pro analyzes them, sorts them into one of the ten categories, extracts information from them, and automatically checks them for compliance against multiple criteria. These criteria were designed by experts with the NMRC of Oncology named after N.N. Petrov and include trial execution, subject selection methods, participant awareness, and more. The LEC tracks the application status in Yandex Tracker, which helps quickly and easily update and adjust application processing and demands no coding background.
Uploaded documents are stored in Yandex Object Storage, within a Yandex Cloud user’s on-premise infrastructure, while data from the application (information about users, applications, comments, and statuses), in Yandex Managed Service for PostgreSQL. To deploy the application and structure the document processing, the experts used Yandex Managed Service for Kubernetes®.
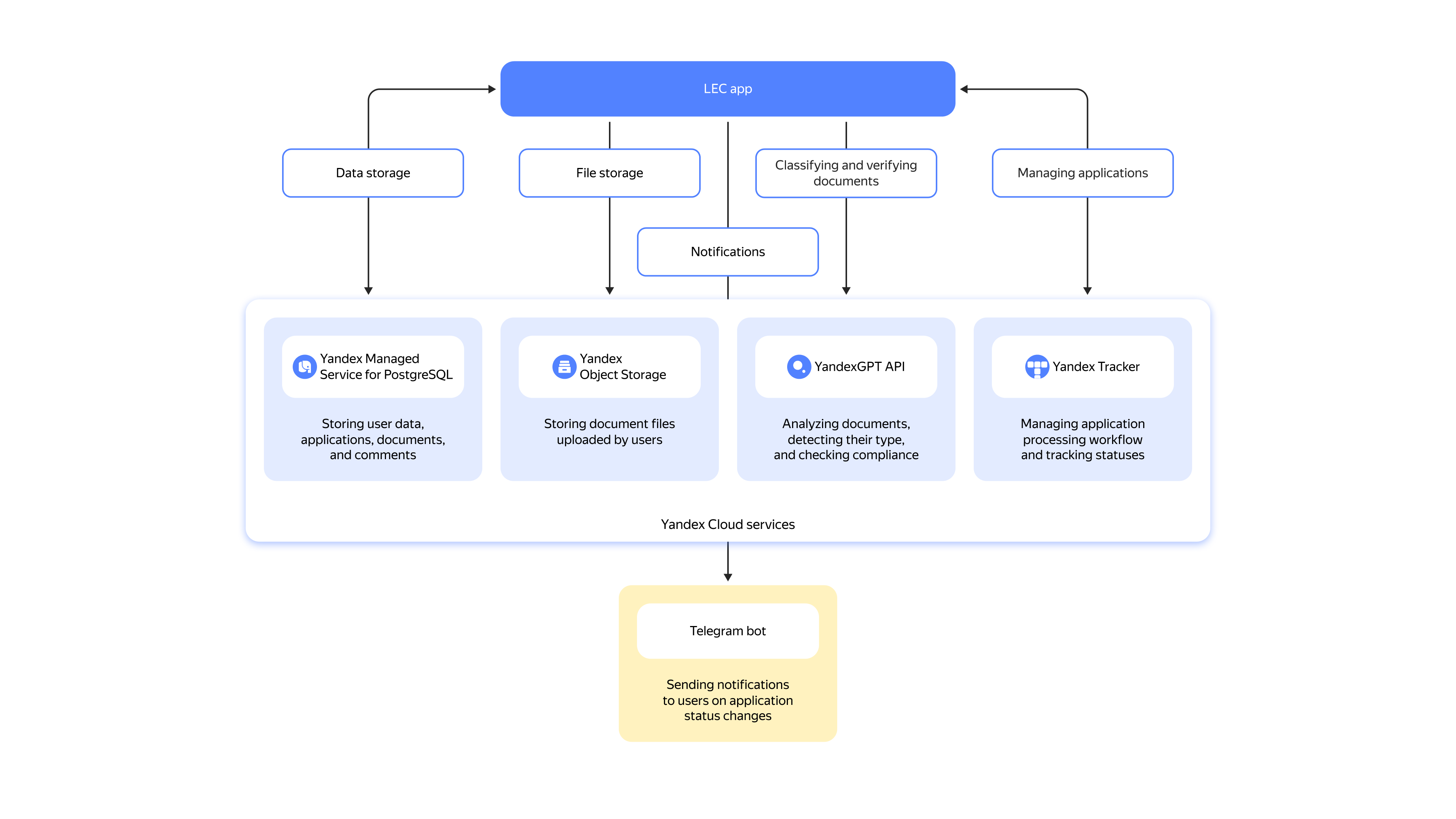
Integration with Yandex Cloud services
Document review is a two-step process. First, the system attempts to define under which category out of the ten the uploaded document falls. After the full document batch has been collected, each document undergoes a criteria compliance check. Each document type has its own criteria. Based on the check results, the user gets a list of suggestions as to what should be fixed in the application. If there is nothing to fix, the application goes to the next stage, at which an LEC expert reviews it.
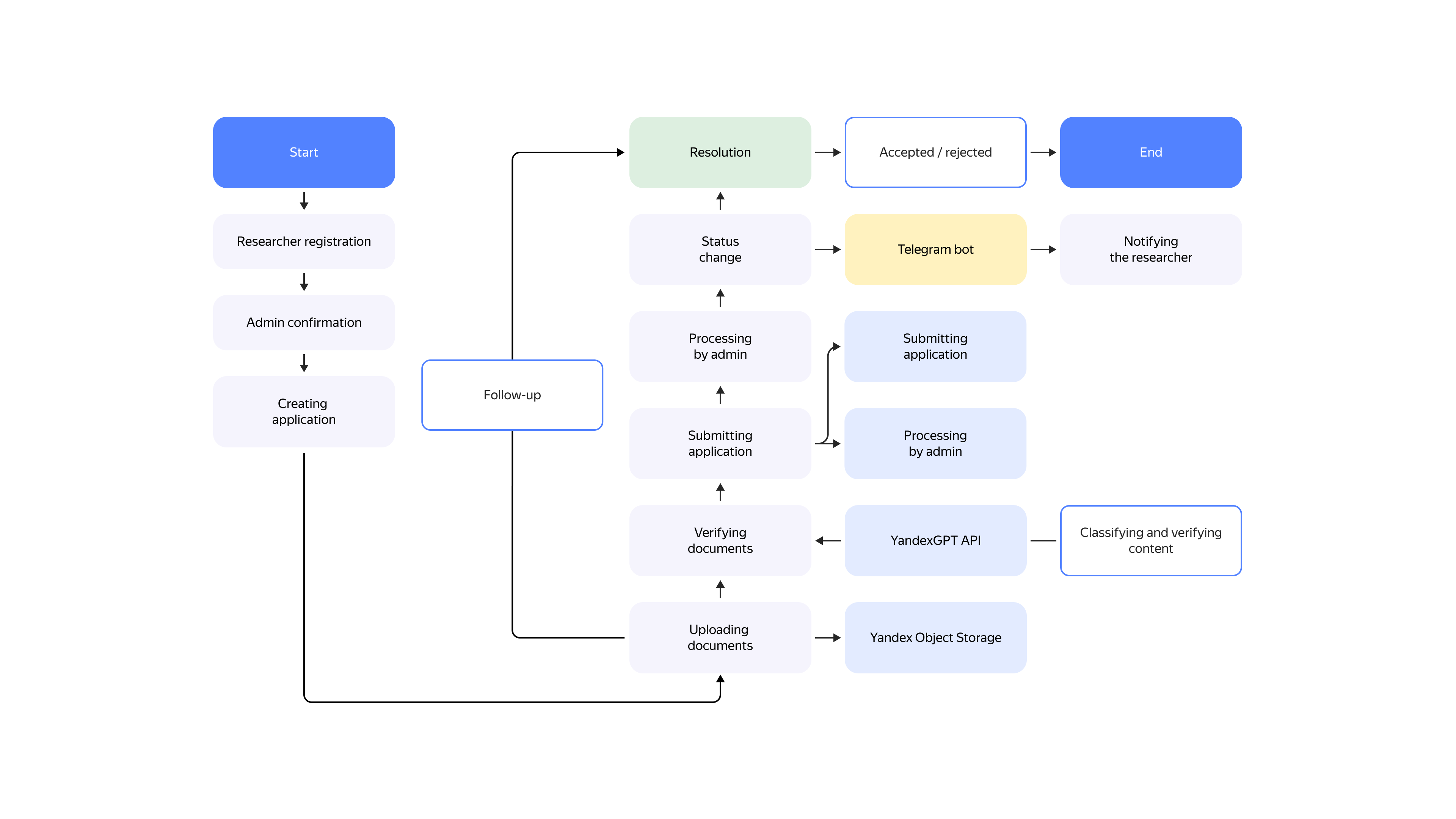
How the solution works
“We use a classifier based on structured output, with a Pydantic structure at input and regular expressions applied to extract key features. Depending on the document type, an individual checklist is generated and included in a prompt for YandexGPT 5 Pro. Based on that prompt, the model creates a final output. The model needed no fine-tuning.
The biggest challenge was documents spanning over up to 300 pages, some with illegible tables. While the system handles text well enough, tables required a dedicated approach involving their conversion into structured data eligible for analysis.”
In 95% of cases, no human effort is required in document review. The expert only needs to make sure the data is correct and take one of the three actions: approve the application right away, make corrections, or reject it.
The solution has been adopted by the LEC at the NMRC of Oncology named after N.N.Petrov:
“Every clinical trial application is hundreds of pages’ worth of tables, charts, treatment descriptions, and other potentially life-saving data. There is no room for error in terms of safety, ethics, or international standards. Processing all that manually would take weeks of hard work. The expert would virtually get lost in papers. Thanks to YandexGPT, this is now a matter of minutes. We have seen this finalization process, which used to take months, conclude in one or two weeks. This is not just about acceleration; this is about extra time the patient gets.”
We are still actively working on this project. Other oncology centers based in BRICS countries and organizations engaged in clinical trials intend to adopt this solution as well. Banks, insurance companies, and state agencies can also employ this tool to process any multi-page documents.
The Yandex Cloud Center for Technologies and Society implements socially significant projects in the fields of education, science, healthcare, environment, and culture. If you have a similar project, please fill out an application.



